
Knowledge sharing and public engagement are critical for finding solutions to the cascading crises caused by the SARS-CoV-2 pandemic. This article is part of an ISC blog series, which aims to highlight some of the latest COVID-19 related publications, initiatives and findings from ISC Members.
Originally published by the Australian Academy of Science

Author: Professor Dianne Nicol
Director, Centre for Law and Genetics,
Faculty of Law, University of Tasmania

Author: Associate Professor Jane Nielsen
Member, Centre for Law and Genetics,
Faculty of Law, University of Tasmania
Among the many issues raised during the COVID-19 pandemic, there is increasing attention on how intellectual property rights, particularly patents, are put into practice. For example, one of the conspiracy theories currently in circulation claims that the novel coronavirus itself is patented, and therefore the patent holder will profit from any cure developed for the pandemic. This is by no means true. Yet issues involving intellectual property rights, in all their guises, litter the COVID-19 landscape, and patents have the most significant potential to fuel discussion and debate in the coronavirus response.
Patent rights exist everywhere around viral pandemics, as seen in simple Google searches for face masks, ventilators, therapies, diagnostics, and vaccines. Globally, many of these patents remain active, and many new applications are being filed. A recent analysis identified hundreds of patents associated with SARS and MERS that, the authors claim, could be relevant in the COVID-19 context.
The rationale for patents is that they encourage innovation. By granting temporary time frames of exclusivity around uses of new technologies, innovators are encouraged to embark on the process of commercial development. Yet questions abound: how new must the technology be? How much exclusivity should be granted, and for how long? What is the extent of the advantage for exclusivity? What uses might be allowed? And when might it be appropriate for governments to step in and take away or modify the rights they have given?
These questions have been the subject of ongoing academic and policy debate for decades, but they are especially relevant now during this global emergency.
By law, there is potential for the Australian government to step in and use patented technology ‘for the services of the state’, or to licence other providers to do the same. Although our Patents Act 1990 allows such practices, they are rarely, if ever, used. Government intervention is probably unnecessary for face masks and other forms of personal protective equipment (PPE), but restrictions on the ability to repurpose patented therapeutics and to develop vaccines raise more serious concerns.
Pharmaceutical companies have always guarded their patents over new chemical and biological medicines. Their rationale is that the high expense and risk of taking therapeutics through the regulatory hurdles to prove they are safe, effective and useful means there needs to be a period of exclusivity once they are on the market. Difficulties will arise if patent holders refuse to permit repurposing investigations to be undertaken by others. This is particularly concerning now, given that many of the patented chemical or biological medicines for the treatment of viral infections such as SARS, MERS, influenza, HCV, and Ebola may be suitable for repurposing.Many of the patented chemical or biological medicines for the treatment of viral infections such as SARS, MERS, influenza, HCV, and Ebola may be suitable for repurposing.
However, some of the major patent holders are taking the pragmatic approach of temporarily suspending enforcement of their patent rights for the duration of the pandemic. In some countries, such as Germany, Israel, Chile and Canada, governments are taking pre-emptive steps to ensure that uses for COVID-19 purposes remain open. A group of scientists and organisations have pledged to make their intellectual property free of charge for use in COVID-19 research. Not all patent holders are taking this benevolent approach and organisations such as Médecins Sans Frontières (Doctors Without Borders) are calling for others to do the same.
With vaccine development, the need to act quickly, and the consequences of not doing so, could be significant. Although there is no evidence yet that patents are being used in ways that might delay COVID-19 vaccine development in Australia, we should not be complacent. The World Health Organization (WHO) has prioritised the accelerated development of vaccines and established the COVID-19 Solidarity Response Fund, which will rely partly on philanthropic and public funding. It is estimated that the cost of developing a COVID-19 vaccine is in the vicinity of US$2 billion. To contribute to the global effort of developing a COVID-19 vaccine, the World Bank and the Coalition for Epidemic Preparedness Innovations (CEPI) recently launched a COVID-19 Vaccine Development Taskforce, which the University of Queensland was invited to join.
Globally we are seeing huge research efforts being poured into COVID-19 treatments and vaccine research. The number of articles related to COVID-19 published in scientific journals has steadily increased since the outbreak of the virus, with many of these proving to be highly influential in the quest for a vaccine. WHO promotes sharing of research data to ensure vaccine development remains in the public sphere. Patent ownership of rights to vaccine development and distribution would conflict with this and other public initiatives.
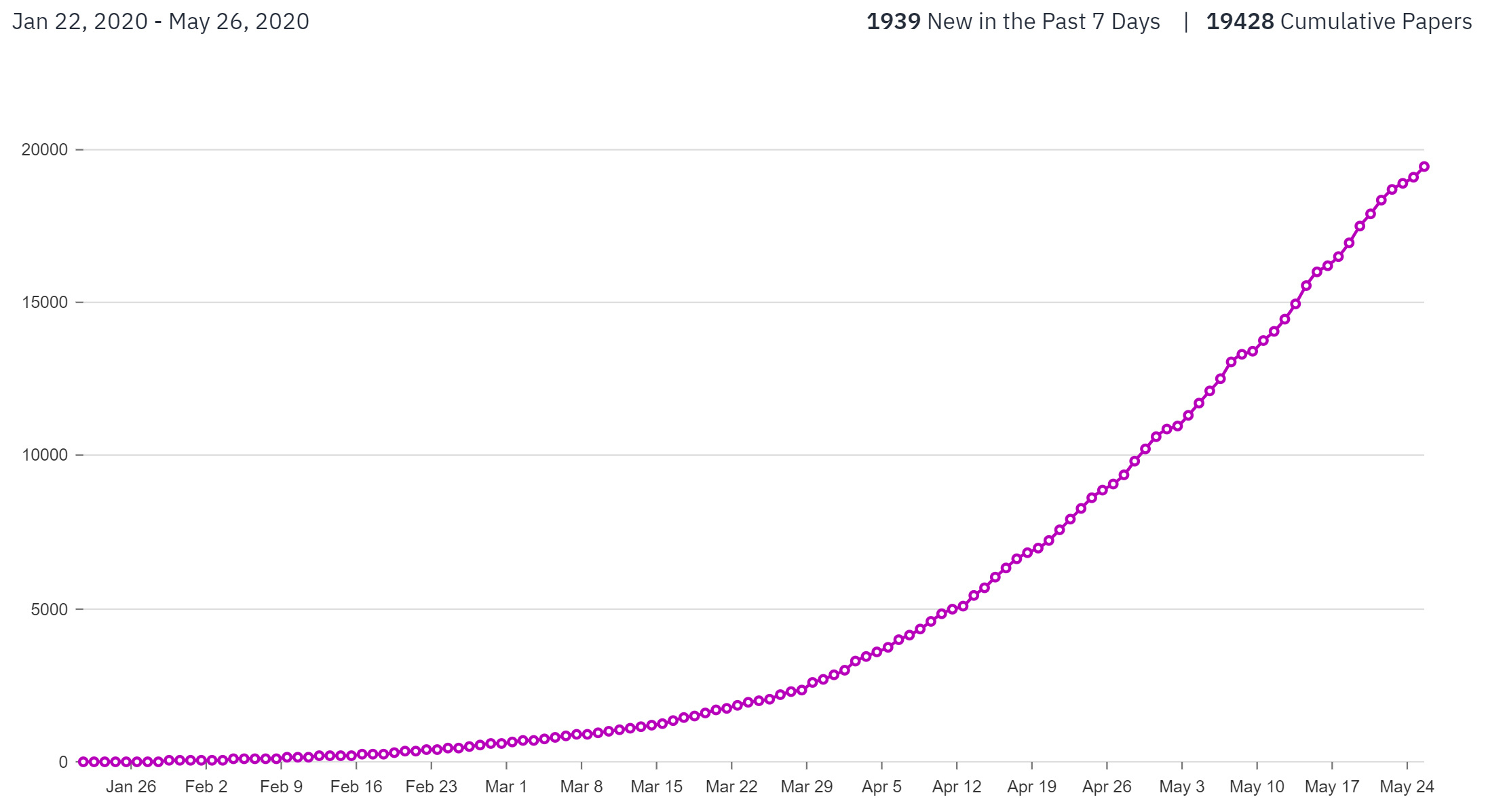
As of 26 May 2020, 16,819 new published papers and preprints were released mentioning ‘covid-19 OR sars-cov-2 OR 2019-nCoV’. Courtesy of Primer AI
Few specific COVID-19-related patent applications have been filed to date in Australia or anywhere else, but based on previous vaccine races this seems inevitable. WHO and the World Intellectual Property Organisation report that large numbers of patent rights have been fragmented across disparate parties, each seeking to claim rights over the various components that make up vaccines.
The current discrepancy between the amount of public funding available and the cost of developing effective vaccines highlights the likelihood that private entities will become competitive players in the race for a vaccine and will subsequently seek to recoup their investment. This push to patent is not surprising given the substantial investment required for development.
One consequence of patents is that competitors may be blocked from developing new vaccines using patented technologies. Although it may be possible to negotiate licences to patents, this process will hinder the speed at which vaccine development can occur. The question is how to overcome this incentivisation gulf during the current pandemic?
To help understand which of the various options may work for COVID-19, we can learn from the approaches taken in previous viral outbreaks. While WHO coordinated a concerted effort to develop a vaccine during the SARS outbreak, many of the parties involved filed patent applications. This led to a plan to ‘pool’ all patents essential to SARS treatments or vaccines—the idea being that users of the pool would be able to collectively license all patents essential to the development of vaccines and treatments at a reasonable price. Early control of the outbreak meant the patent pool never eventuated.
Other outbreaks have prompted similar initiatives. Unitaid (a global health initiative) and WHO initiated a patent pool to centralise a number of essential patents relevant to HIV/AIDS treatment. This arrangement evolved into a pool containing many patents related to HIV, Hepatitis C and tuberculosis.
Setting up prize mechanisms to fund important prospective scientific research is another solution that has been explored in the wake of public health crises. An alternative method is to promote public–private partnerships that aim to encourage broad access to end products. This requires the agreement of patent holders not to assert their patent rights against certain parties, including those in other countries.The scale of the pandemic and vaccine requirements have the potential to change the landscape around the patenting of vaccines.
Ultimately, effective vaccine development for COVID-19 depends on a concerted commitment to share research, clinical trials data and virus samples. Supporting this, researchers are making their findings openly available, which assists with the momentum of the united global response. This issue arose during the H5NI (bird flu) outbreak, when an Australian company patented an H5NI vaccine derived from Indonesian samples donated to the WHO Influenza Network. The Indonesian Government was then unable to access vaccine supplies when the disease struck Indonesia. The ensuing publicity resulted in the patent holder announcing it would license at no cost. The possibility that the Australian Government might step in is also a likely incentive to voluntarily license.
The SARS pandemic aside, previous global efforts at developing vaccines have lacked the compelling urgency of the current COVID-19 research push. The severity of this pandemic, the likes of which the world has not seen for 100 years, has mobilised the global research community in the quest for a vaccine. Recently CSIRO was funded by CEPI to test two promising vaccine candidates. The US Government has invested over US$400 million (with additional industry support) to fund the development of promising vaccine candidates by two pharmaceutical companies. Seasonal flu vaccines are generally incentivised through public funding in conjunction with WHO. However, the reality is that the financial involvement of the private sector will be essential to complement public sector research to move COVID-19 vaccines rapidly to the clinic.

Although a pandemic of this scale was anticipated, its ferocity has shocked health authorities. The scale of the pandemic and vaccine requirements have the potential to change the landscape around the patenting of vaccines. In line with broad principles of sharing, the Australian research effort should be guided by the international effort and support and complement international research priorities. Australian Government commitment to assist will help ensure equitable access to vaccines once developed: Australia, as other countries, must commit promptly to contributing toward a shared research and funding effort. The government must also be prepared to use all of the regulatory tools available to it, should there be non-compliance with the spirit of cooperation by individual entities. Private property rights have a limited place in global health crises of this nature.
This topic’s links to the Sustainable Development Goals:

This feature article from the Australian Academy of Science is part of the ‘Science for Australians’ series where experts are asked to shed light on how science benefits all Australians and how it can be used to inform policy.
Views expressed in this feature remain those of the authors.
The authors declare no conflicts of interest.
This article has been reviewed by the following experts: Professor Michael Wallach School of Life Sciences, University of Technology Sydney; Professor Mark Perry School of Law, University of New England
© 2020 Nicol and Nielsen. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.